You can
put this solution on YOUR website! .
How much water should be added to 12 mL of 8% alcohol solution to reduce the concentration to 6%?
~~~~~~~~~~~~~~~~~~~~
Your original solution contains 0.08 * 12 = 0.96 mL of pure alcohol.
You want to have the new total volume V such that 0.96 mL of pure alcohol
make the concentration 0.06.
Write an equation saying that 0.06 of V is 0.96
0.06*V = 0.96.
Solve it and find V
V = 0.96/0.06 = 16 mL.
So, the original volume is/was 12 mL; new volume should be 16 mL.
Hence, 16 - 12 = 4 mL of water should be added.
ANSWER. 4 mL of water should be added.
CHECK. 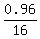 = 0.06, correct.
= 0.06, correct.
Solved.