Question 1186275: A solution was prepared by dissolving 25g of KOH in 250cm3 of distilled water.
Calculate the concentration of the solution in mol dm-3
[K=39, O=16, H=1]
Found 2 solutions by josgarithmetic, ikleyn:
Answer by josgarithmetic(39618)   (Show Source): (Show Source):
Answer by ikleyn(52794)   (Show Source): (Show Source):
You can put this solution on YOUR website! .
Molecular Weight of KOH (Potassium hydroxide) is 56.
So, 1 mole of KOH is 56 grams.
25 grams of Potassium hydroxide is 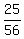 = 0.4464 of a mole.
250 cm^3 = 0.25 dm^3.
The concentration of the solution in = 0.4464 of a mole.
250 cm^3 = 0.25 dm^3.
The concentration of the solution in 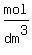 is, THEREFORE, is, THEREFORE,
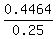 = 1.786 = 1.786 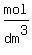 . ANSWER . ANSWER
It is my answer.
----------------
I am not a chemist in my basic education, although learned Chemistry diligently in my high school.
Working on this assignment, I consulted with Wikipedia and other Internet sources regarding properties
of KOH at normal temperature, including solubility.
I also have many years experience working with civil and mechanical engineers, where skills and a common sense
in operating with dimensions are MUST.
I started doing this assignment, because calculations by @josgarithmetic seem to be absurdist to me
and irrelevant to the posed question.
//////////////
After seeing my post, @josgarithmetic did what he does every time: re-wrote the numbers from my post.
So, it was not for nothing, when I worked on my solution . . .
|
|
|